This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
What Kind of Technology Do Life Sciences Companies Rely Upon Most and When?

In many life sciences companies, the strategic value and use of technology changes with the milestones of the business lifecycle. Our survey helped us understand the shifting priorities for relying on technology as life sciences organizations come to a more expansive understanding of what digital tools can help them accomplish. As reflected in survey findings, the industry follows a path of digital enablement that Sikich has long facilitated for the life sciences clients we serve. Below, we consider survey findings that illustrate how life sciences companies view and treat technology, and how we assist them in reaching their goals.
Technology seen as business-critical mostly by larger companies

Life sciences analysts, writers, and industry insiders have often pointed out a disparity in many life sciences organizations: the contrast between the innovation and resourcefulness at work in creating new drugs and treatments to address urgent problems and the more conservative perception and use of digital technology and data. IT was often seen to be a cost center that provides and manages fundamental business functionality. Recently, however, more and more life sciences companies have changed their view of the role technology could or should play. They increasingly look for ways to use technology to evolve their engagement models to create and maintain relationships with investors or meet the needs of payers, patients, and care providers.
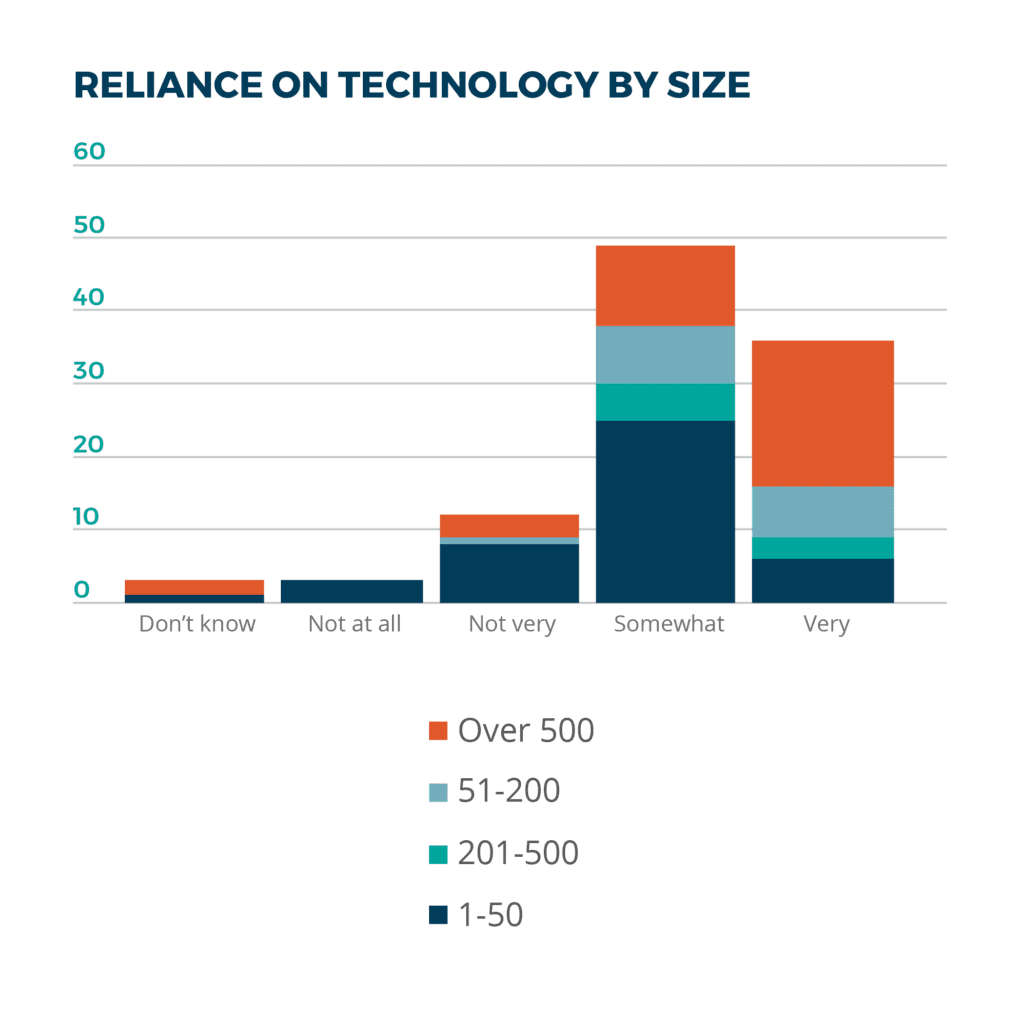
In our survey, it appears that the largest life sciences companies with workforces of more than 500 rely “very heavily” on business systems to run their operations. Most of the smaller firms with up to 50 employees do so “somewhat.” Surprisingly, some companies stated that they don’t rely on technology at all or only minimally.
At Sikich, we find that many early-stage, lean-running life sciences companies take advantage of technology to operate as efficiently as possible. They work with us to implement Sikich SuiteSucces for Life Sciences, a version of NetSuite that is optimized for life sciences businesses, to accelerate, streamline, and automate processes so they can scale without incurring large technology investments as they grow. In addition to SuiteSuccess, they often layer Coupa Business Spend Management software or optical character recognition (OCR) technology to simplify invoice management.
When we work with these clients, we help them plan technology for years to come, in addition to addressing their current concerns. From many successful engagements in the industry, we know how companies can benefit from technology that fits how they work. Decisions made early on can make a big difference when organizations become ready for commercialization or approach events like going public. If life sciences clients imposed restrictions on themselves by adopting technology that cannot meet their changing requirements, we collaborate with them to adjust course before it becomes problematic.
Charting a path for technological maturation
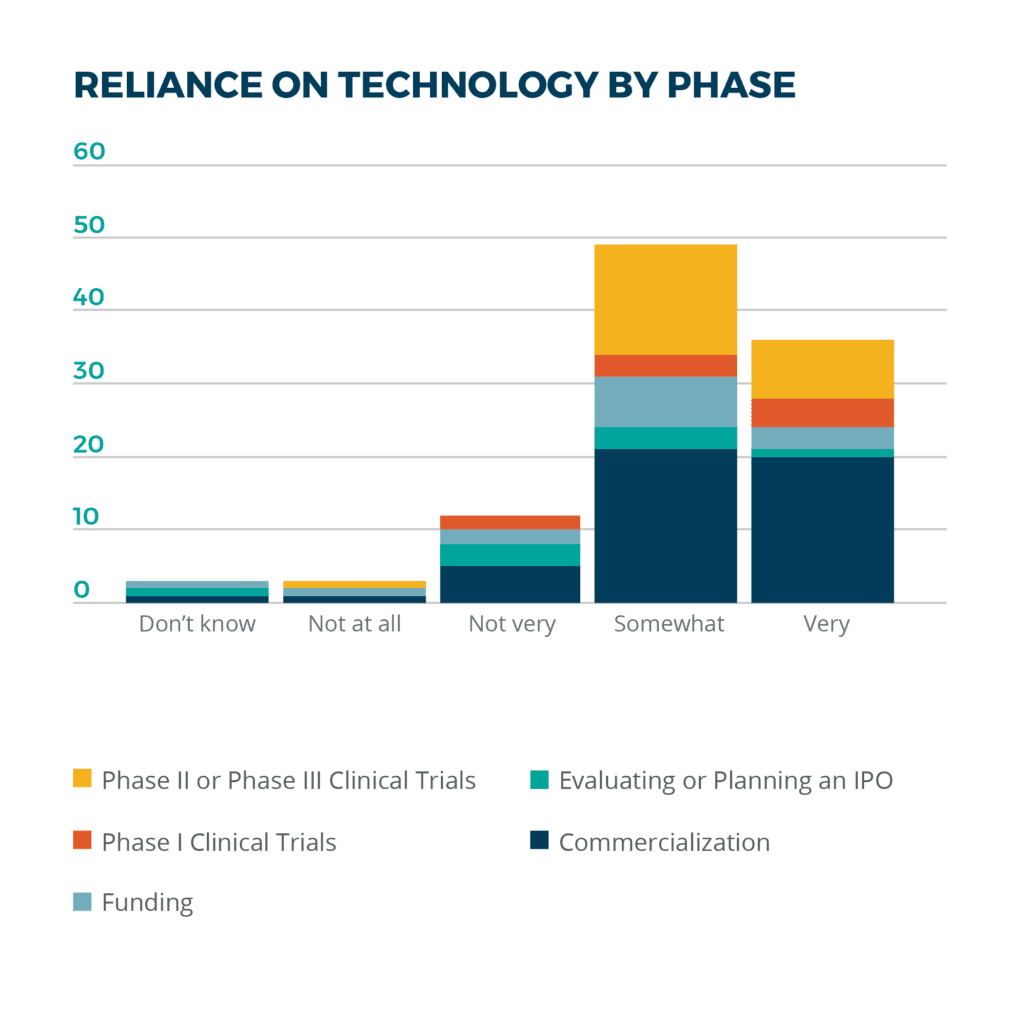
Looking at life sciences companies’ reliance on technology from another perspective, nearly half of the companies at the commercialization stage depend strongly on digital enablement. Thirty-seven percent of the companies were considering or planning an IPO, and 21 percent of those at the funding stage indicated that they rely on technology “not at all” or “not very.” It often happens that life sciences organizations don’t consider technology more strategically until they are close to their IPO or a comparable, major milestone. Our team will engage and assist, but we generally try to start conversations much earlier in order to help companies avoid the inefficiencies and direct and indirect costs of having long outgrown their business software.
A Sikich Business Process Alignment (BPA) is one approach we often use to review and optimize a company’s activities to ensure a smooth journey from start-up to a mature organization. Recently, we performed a BPA for a 300-employee company that had already gone public, but found itself hampered by technology tools like QuickBooks that had served it well in the beginning. Over the years, the business added many additional software tools, including several procurement systems. Data became fragmented and difficult to access, and most team members had to navigate several systems to do their work and move processes forward.
Implementing SuiteSuccess for Life Sciences at an earlier stage and integrating specialized software tools into the ERP system without resulting in a disjointed technology environment would have made the company’s growth and decision-making much easier.
Planning technology to enable quality and vendor study management
Sikich guides companies along proven paths when it comes to two important areas of life sciences operations, quality management and vendor study management. Most of our clients don’t need to be overly concerned with quality management until they work with CMOs to produce drugs and take them through trials. To begin with, they can effectively use the quality management functionality of SuiteSuccess for Life Sciences. As they get closer to commercialization, they may require a specialized software tool like TrackWise. We routinely deploy, configure, and integrate TrackWise with SuiteSuccess.
The Vendor Study Management capabilities in SuiteSuccess for Life Sciences allow life sciences companies to manage their spending with CROs efficiently within the ERP environment. As they grow, it may make sense to add software like Coupa to manage procurement and spending. Sikich consultants know how to introduce these software tools and their data flows into a NetSuite environment to avoid fragmentation and ensure the best visibility of financials and operations for companies’ business roles.
Next steps
To gain more insight and explore technology opportunities for your life sciences organization, you can:
- Download the complete survey report by Pharmaceutical Executive and Sikich.
- See how Sikich supports life sciences companies.
- Read the previous blog post in this series regarding technology for talent and growth in life sciences companies.
- Contact Sikich.
This publication contains general information only and Sikich is not, by means of this publication, rendering accounting, business, financial, investment, legal, tax, or any other professional advice or services. This publication is not a substitute for such professional advice or services, nor should you use it as a basis for any decision, action or omission that may affect you or your business. Before making any decision, taking any action or omitting an action that may affect you or your business, you should consult a qualified professional advisor. In addition, this publication may contain certain content generated by an artificial intelligence (AI) language model. You acknowledge that Sikich shall not be responsible for any loss sustained by you or any person who relies on this publication.




